Water
Intro
A while ago, I was reading a post on Quora, and while I
forgot the exact wording of the question, it was something along the lines of
“Are cells really mainly composed of just water?”. On a side note, for those
who do not know what Quora is, it is a Q&A site that I frequent on my phone
because it uses less data—there are never really any images or videos to load
(I only have 1GB of data). However, if I were to scroll through my Facebook
newsfeed, I get paranoid from all the photos and videos that pop-up as I scroll
down, because then I feel like I am going to go over my data plan.
Anyways, coming back to this question—to be honest, I didn’t
even bother looking at the answers to this particular question, because I
already knew the answer. However, what irked me beyond belief was two words. “Just
water” irked me to the core.
You see, when I was younger, I had the same view of water as
the asker of this question. The problem with water in our society is that we
take fresh, drinking water for granted. Tap water is cheap, so cheap that water
bottle companies shame our tap water to sell water at ridiculous prices. I even
saw a YouTube video about a social experiment about how bad this situation is—essentially
customers would leave the restaurant immediately after being told they would
not be offered a free glass of water. Likewise, if I ever went to a fast food
place when I was a kid, I would scoff and wonder why anyone would buy water
when they could get a soda or juice instead.
Please note that I did not make this post to try to convince
you that water is a healthier choice and that you should drink more of it.
Rather, the biochemistry nerd in me wants to convince you why water, “just
water”, is so crucial for your body that even tap water shouldn’t be treated
like its worthless—it had so many roles in allowing our cells to function
properly.
Membranes
One of the most important jobs of water in our bodies is to aid
in the creation of membranes. A membrane is essentially a barrier that defines
the boundaries of the cell, as well as further compartments within the cell
itself—they prevent most compounds from going in and out freely. The cell then uses
proteins embedded within the membrane to control whether certain compounds can
pass through or not.
Water is crucial because membrane formation relies on the
hydrophobic effect. In layman terms, the hydrophobic effect is the reason that
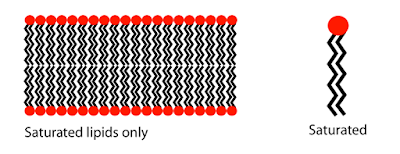
water and oil don’t mix, because they don’t like each other. If you look at the
figure below, you can see that membranes are composed of these compounds call
phospholipids (diagram on the right). Essentially the red circle, represents
the “head”, while the black part represents the “tails”. The “head” likes to be
around water, while the “tails” do not. Thus, the head likes to face water,
while the tail likes to be in the surroundings of other tails. For this reason,
these phospholipids form this sort of membrane as seen below (diagram on the
left). This is called a phospholipid bilayer. More specifically, this process
has to do with entropy and ground state energies, but I won’t get into that
here.
Membranes - Mitochondria – The Powerhouse of the Cell
Earlier, I was pretty vague as to the benefits of membranes,
outside of it being what keeps the cell contents within the cell (the plasma
membrane). For this reason, I would like to discuss one of the most important uses
of membranes by discussing mitochondria, a.k.a. “the powerhouse of the cell”. I
remember doing that BuzzFeed science quiz a long time ago where all of the
answers were “mitochondria are the powerhouse of the cell”. Without membranes
in the mitochondria, it would be unable to make energy, and thus your cells would
die, and you would die.
Mitochondria produce energy in the form of a compound called
ATP (adenosine triphosphate). Other parts of your cells use the energy released
from breaking this ATP into the more stable ADP (adenosine diphosphate) + Pi
(inorganic phosphate) to perform all sorts of functions in your cells. For
example, for neurons to send signals, potassium and sodium ions needs to be
pumped in order to create a gradient—this requires energy from ATP.
I now claim that without mitochondria being in a water solvent
environment, essentially no ATP could be produced.
Note that when glucose first enters you cell, the first
process it goes through is called glycolysis. This step converts this one
glucose molecule into two molecules of something called pyruvate. During this
process, there is a net gain of 2 ATP (4 ATP are made in this process, but 2
ATP are consumed in the process). Glycolysis does not require a membrane to
function. Interestingly, yeast cells and certain other organisms can rely
solely on this process, in which fermentation occurs (for reasons I will not go
into, fermentation is a necessary step in environments that lack oxygen, as the
ETC discussed below requires oxygen to function).
Once pyruvate has been made in the cytosol, it is transported
into the mitochondria, where it is first converted to Acetyl-CoA, before
undergoing the Krebs cycle. In this process, energy storing compounds called
NADH and FADH2 are created, and these compounds enter the Electron
Transport Chain (ETC). The diagram below outlines these steps.
Now the meaty part of the importance of membranes resides in
the Electron Transport Chain. This process generates quite a bit of ATP, in the
range of 30 ATPs per one pyruvate molecule. This pales in comparison to the amount
of ATP generated by glycolysis (a measly 2 ATP per glucose molecule).
As seen by this diagram from Wikipedia, the electron
transport relies on the existence of membrane, which are the parts that look
like a giant accordion. Essentially, the energy that was transferred into the
making of NADH and FADH2 from the Krebs (a.k.a. citric acid cycle) cycle are
released, and protons (H+ in the diagram), are pumped into the intermembrane
space.
I like to think of this as a giant dam. Essentially, H+ is
being pumped out of the mitochondrial matrix into the intermembrane space, and
its only way to get back is to pass through ATP synthase. ATP Synthase is the
part of the damn that harnesses energy due to water pressure, using this energy
to convert ADP + Pi back into ATP. But instead of water, it is with hydrogen
ions. The proteins that pump these H+ ions into the intermembrane space act as
the river that keeps the damn going.
Note that without the existence of membranes, the pumped H+
ions could re-enter the mitochondrial matrix without passing through ATP
synthase, which means ATP is not being made through this process. It is a known
fact that if a hole is created in the inner mitochondrial membrane, which
allows for the free flowing of H+ ions, the cell will die pretty much
instantly.
Proteins
To further quantify to you the important of water in our
cells, I want to turn to proteins.
I am sure you are already familiar with the code of DNA,
that being A, C, G, and T (adenine, cytosine, guanine, and thymine
respectively). Interestingly, proteins also have a code, of which each “letter”
of the code is called an “amino acid”. Amino acids all have the same backbone,
but their side chains all vary. In humans and most other organisms, there are
only 20 amino acids to choose from.
Essentially every three letters from the DNA code for one
amino acid, and the ribosomes are responsible for recruiting free floating free
amino acids and assembling the proteins based off this code. Many variations of
letter codes may code for the same amino acid, as this process allows for mutations
in DNA to have less of a detrimental effect on the survival of both the
individual and the species.
It is important for understand that proteins are designed to
fold properly in the environment they are in. Excluding membrane proteins, most
proteins are designed to fold in the presence of water—they actually start
their folding process while the ribosome is still elongating the amino acid
chain. Because there are only 20 distinct amino acids to choose from, while a
protein can consist of thousand of amino acids, it is the final distinct shape of the protein
that defines its function—there are not enough distinct amino acids for each
protein to have its own “special one”.
As I mentioned in the membrane section, proteins fold due to
the hydrophobic effect as well. Because all amino acids share a common
backbone, it is the side chain that determines the properties of that specific
amino acid (are represented by R1, R2, and R3 in the diagram below). It is the
hydrophobic amino acids that like to remain on the inside of the protein, with
the polar residues facing the outside. Proteins rely mainly on the hydrophobic
effect to ensure proteins fold in a predictable way. While there are other
factors that determine how a protein will fold, such as pH, and the actually “jig-saw”
shape of the amino acids itself, the hydrophobic effect is perhaps one of the
most important determinants of protein shape, and thus protein function.
DNA
Finally, the stability of DNA is in part due to the hydrophobic
effect as well. The bases involved (A, G, T, and C) are mainly hydrophobic.
Thus, these nucleotides can stack efficiently upon each other, promoting DNA
stability.
Note that I did not mention mRNA (messenger RNA), which is
made by RNA polymerase from the DNA template strand. While ribosomes use mRNA to
translate into proteins, there is no concern about its stability, as mRNA is meant
to degrade quickly (it is just a copy).
Conclusions
I hope that this post shows why water is so important to all
of our cells, and I hope you have a greater appreciation for why we cannot live
for long without water in our bodies. There are obviously more reasons in human
biology (i.e. anatomy) as to why water is important, such as for excreting waste,
etc., but I just wanted to offer my knowledge as to the importance of water in
each individual cell.
Finally, I hope that this post will give you some more respect
for tap water, because it is just as beneficial to you are the bottled stuff.




Comments
Post a Comment